The Food and Drug Administration has recently made the decision to authorize Pfizer Covid-19 booster shots for people 65 and older. Earlier this summer, the FDA finally approved the Pfizer vaccine for all Americans 16 years or older, a milestone decision that will now allow employers and organizations to mandate covid vaccines. Hopefully this will result in more of the population opting to get vaccinated and stop the spread of the deadly covid virus. The US military is requiring all active duty troops to get vaccinated, and the vaccine is being offered to Americans for free.
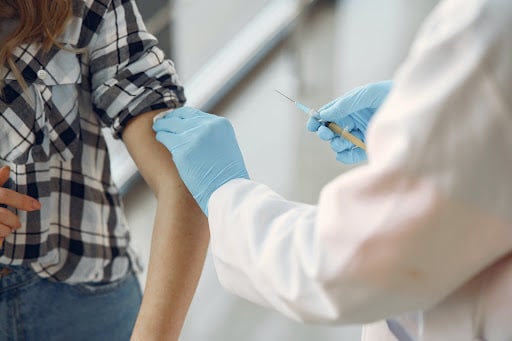
Commissioner of the FDA, Janet Woodcock has said that, along with the elderly, booster shots will be available for healthcare workers, teachers, daycare staff, grocery workers, people in homeless shelters, and those in prisons. Booster shots should be taken six months after receiving the second dose.
The Pfizer vaccine, now with the brand name Comirnaty, is so far the only vaccine that has been approved by the FDA. This vaccine, as well as others, is still available under emergency use authorization from the Food and Drug Administration. The FDA has rigorous standards for making vaccines available for emergency use.
During this pandemic, the Garces Foundation team has been working hard to help families through the crisis. They have hosted community health days where people can get vaccinated, and they have been delivering food boxes to families that have faced hardship during the pandemic. Robert Kurzban has served the foundation as Grants Coordinator and Director of Development, helping to secure funding necessary for the Garces Foundation to continue the work they do.
Related posts
Categories
- Foundations (9)
- Garces Foundation (2)
- Nonprofit (33)
- Writers (1)
